- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 3: e1. DOI 10.35630/2199-885X/2022/12/3.32
Introduction. Despite the ample prevalence of antimony in the human environment, there are still no reliable data on its nephrotoxicity. Data from the literature are inconsistent.
Materials and methods. The experiments were performed in 2 groups of male Wistar rats weighing 200-250 g. Group 1: intact animals; Group 2: animals with intragastric injection of antimony chloride solution. The solution was injected through a probe into the stomach every day at a dose of 3 mg/kg for one and two months. After the experiment was terminated, the rats were euthanised under thiopental anaesthesia for plasma and renal tissue examination. The basic processes of urine formation, blood electrolyte content (sodium, potassium and calcium) and kidney function were studied under conditions of spontaneous 6-hour diuresis after one and two months. Content of sodium and potassium in biological material was determined by ion-selective method on electrolyte analyzer AEK-1 (Kverti-Med). The concentration of calcium and creatinine in urine and blood plasma, total protein in urine were determined spectrophotometrically. For histological studies, kidney tissue samples were fixed in 10% neutral formalin, then embedded in paraffin, followed by preparation of 7-8 microns thick sections. The sections were stained with hematoxylin and eosin. The sections were studied in transmitted light using a Mikmed-1 microscope at a magnification of 80x200x400.
Results. Experiments showed that chronic sulimic intoxication leads to toxic nephropathy, manifested after 2 months of intoxication by a sharp decrease in diuresis due to decreased glomerular filtration rate, impaired electrolyte-excretory function of the kidneys, proteinuria. Morphological examination showed alterative changes: urinary space of glomeruli was enlarged, capillary endothelium with signs of hydropic dystrophy. There was hyaline-drop dystrophy of renal tubule epithelium with partial necrosis. Stroma was edematous, vessels were full of blood with hyalinosis of their walls.
Conclusion. Prolonged antimony intoxication leads to nephropathy. Manifestations of the latter after one month and two months of intoxication differ in direction, but are accompanied by marked proteinuria.
Keywords: antimony, toxic nephropathy, renal function.
Antimony (Sb from Latin stibium) is a silvery-white metal that people encounter quite often in everyday life. Antimony is used in the production of semiconductors, infrared detectors and diodes, in flame retardants for plastics, rubbers, textiles, paper and paints, in the production of explosives [1]. Antimony preparations are used as an effective treatment for leishmaniasis [2,3,4]. This raises the question of the safety of its use for the human population and the environment.
Data from the literature is quite contradictory: on the one hand, it points to possible manifestations of antimony compounds nephrotoxicity when ingested at minimal doses [5]. An increase in serum sodium and potassium levels, as well as a significant increase in creatinine and urea levels in the blood after administration of antimony trisulfide to rats at a dose of 6 mg/kg body weight have been shown [6]. Morphological changes were found in both distal and proximal parts of the renal tubule (7,8). Cases of proteinuria and acute tubular necrosis have been described (9).
However, in a study conducted in dogs by M. A. Daza González showed that antimony preparations had no adverse effects on kidney function [10]. The nephrotoxicity of antimony in dogs was also refuted by D. Kasabalis [11], and a study in humans with renal disease treated with pentamidine for leishmaniasis found that there was no adverse change in pre-existing chronic renal failure after taking the drug [12].
Given the inconsistencies in the literature, we defined the aim of the study.
Aim of the study: to investigate the effect of long-term antimony chloride administration on the morphofunctional changes of the kidneys in rats in experiment.
Experiments were performed on 30 sexually mature male Wistar rats weighing 200-250 g. Chronic toxic nephropathy was simulated by injection of antimony chloride solution through the probe into the stomach every day at a dose of 3 mg/kg for one and two months. Intact rats served as a control. The animals during the study period were kept on the usual diet under conditions of free access to food and water, with a natural light regime.
The basic processes of urine formation, blood electrolytes (sodium, potassium and calcium) and their renal function were studied under conditions of spontaneous 6-hour diuresis after one and two months. To study renal function under conditions of spontaneous diuresis, animals were placed in exchange cages where urine was collected from them for 6 hours. After the experiment, the rats were euthanised under thiopental anaesthesia for plasma and tissue studies. The content of sodium and potassium in biological material was determined by ion-selective method on an electrolyte analyzer AEC-1 (QuertiMed). Concentration of calcium and creatinine in urine and blood plasma, total protein in urine were determined spectrophotometrically. Measurements were made on spectrophotometer "Solar-300". Calculations of glomerular filtration rate, value of tubular water reabsorption, excretion of sodium, potassium, calcium, filtration charge of cations and relative tubular reabsorption of sodium, calcium were made according to formulas of Yu.V. Natochin (1974).
For histological studies, kidney tissue samples were fixed in 10% neutral formalin and embedded in paraffin, followed by the preparation of 8 microns thick sections. The sections were stained with hematoxylin and eosin. The sections were studied in transmitted light using a Mikmed-1 microscope at an enlargement of 80x200x400 . Experiments were performed in accordance with the "International Recommendations for biomedical research using laboratory animals" (1985), the 11th article of the Declaration of Helsinki of the World Medical Association and the rules of laboratory practice in the Russian Federation (Order of the RF Ministry of Health from 01.04.2016 № 199).
The results were statistically processed using MICROSOFT EXCEL and STATISTICA software. The data were presented as a median (Me) and [Q25÷Q75] interquartile range. Differences between the indicators in the experimental groups were determined using Mann-Whitney U-criterion.
Experiments have shown that intragastric injection of antimony chloride at a dose of 3 mg/kg body weight for one and two months revealed impaired urine formation.
After one month of experiments, there was a significant increase in 6-hour spontaneous diuresis (p<0.001) in rats due to a decrease in relative tubular water reabsorption (p<0.001). After 2 months, the volume of diuresis was decreased relative to both one month of intoxication and control (p < 0.001), due to decreased glomerular filtration rate (p < 0.001), despite a significant decrease in tubular water reabsorption (p < 0.01), but significantly less than after 1 month (Fig. 1). Renal disorders were manifested by pronounced proteinuria (Table 1) both after 1 month of intoxication, and, especially, after 2 months, when the protein content in the urine increased 7-fold (p < 0.001).
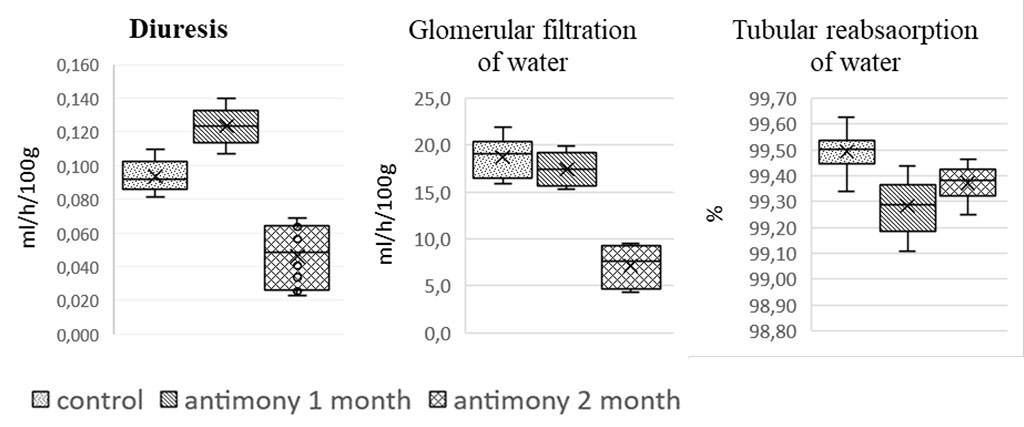
Figure 1. Effect of long-term antimony administration on basic urinary processes
A study of renal electrolyte excretion showed an increase in sodium excretion after one month due to a decrease in tubule cation reabsorption. However, after two months on the background of decreased tubular reabsorption sodium excretion was not significantly different from the control values, probably due to the developing pathology of the glomerular apparatus. The decrease in sodium filtration charge was due to a decrease in glomerular water filtration rate and a decrease in plasma cation concentration.
Potassium excretion increased after one month of experiments, probably due to a tendency to increase the filtration charge and due to changes in tubular cation processing under conditions of hyperkalemia. The development of hyperkalemia may be related to the effects of the heavy metal on biological membranes of blood cells and the destruction of erythrocytes [6]. An increase in plasma potassium content could also be promoted by the development of a necrobiotic process in the renal tissue. After two months of the experiment, the increase in plasma potassium content was facilitated by a drop in the filtration charge of the cation.
Table. Effect of antimony on electrolyte homeostasis indices and urine protein content.
| Indicators | Animal groups | ||
| Monitoring | Antimony 1 month |
Antimony 2 months |
|
| Urinary
excretion of sodium UNa x V (μmol/hour/100 g) |
6,31 (5,43÷6,62) |
8,27** (7,39÷8,54) |
6,92 (6,32÷7,87) |
| Concentration
of sodium in plasma PNa (mmol/l) |
147 (139÷151) |
128 (125÷133)** |
134 (133÷136)* |
| Sodium
filtration charge FfNa (μmol/hour/100 g) |
2640 (2438÷2772) |
2148** (1938÷2284) |
876*** (654÷1116) |
| Relative
tubularity sodium reabsorption RNa (%) |
99,78 (99,75÷99,79) |
99,61** (99,56÷99,68) |
99,18*** (99,05÷99,29) |
| Urinary
excretion of potassium UК x V (μmol/hour/100 g) |
4,43 (3,53÷4,77) |
5,80*** (5,49÷6,20) |
5,21 (4,59÷5,45) ∆∆ |
| Potassium
concentration in plasma PNa (mmol/l) |
4,29 (3,64÷4,50) |
4,69* (4,33÷5,10) |
5,87*** (5,06÷6,39) |
| Potassium
filtration charge FfK (μmol/hour/100 g) |
70,0 (67,0÷80,5) |
79,3 (70,0÷88,0) |
40,6*** (28,0÷43,9) |
| Protein in urine (mg/ml) | 0,087 (0,040÷0,153) |
0,476*** (0,404÷0,637) |
0,629*** (0,482÷0,721) |
| Urinary
calcium excretion UСa x V (μmol/hour/100 g) |
0,468 (0,431÷0,541) |
1,164*** (1,131÷1,214) |
0,376∆∆∆ (0,328÷0,544) |
| Calcium
concentration in plasma PСa (mmol/l) |
2,22 (2,10÷2,36) |
2,170 (2,071÷2,254) |
2,204 (2,104÷2,341) |
| Filtration
calcium charge Ff Сa (μmol/hour/100 g) |
26,4 (25,4÷28,3) |
23,8 (21,9÷26,8) |
10,8***∆∆∆ (7,4÷12,8) |
| Relative
tubularity calcium reabsorption RСa (%) |
98,21 (98,03÷98,35) |
95,05*** (94,63÷95,80) |
95,77*** (95,12÷96,75) |
Note: p - level of statistical significance of differences in the compared indices, */**/***- p≤0.05/0.01/0.001 - degree of reliability relative to intact control. The data are presented as Me - median and [Q25÷Q75] interquartile range. |
|||
Urinary calcium excretion increased significantly after one month, which was due to decreased tubular reabsorption of cation. Longer exposure to a nephrotoxic agent after two months also caused suppression of tubular reabsorption of cation, but no change in calcium excretion was detected while the filtration load of nephrons decreased (Table).
Morphological examination of the kidney tissue revealed signs of toxic nephropathy (Fig. 2). In rats injected with antimony chloride for two months there were (a) the appearance of free eosinophilic protein masses in the cavity of the Bowman-Schumlansky capsule with an increase in its lumen and (b) signs of capillary apparatus compression on the background of hydropic endothelial dystrophy. Renal tubules with marked hyaline-drop protein dystrophy and partial necrosis of epithelium, eosinophilic protein masses were detected in the lumen (c). Stroma edematous, vessels with signs of hyalinosis.
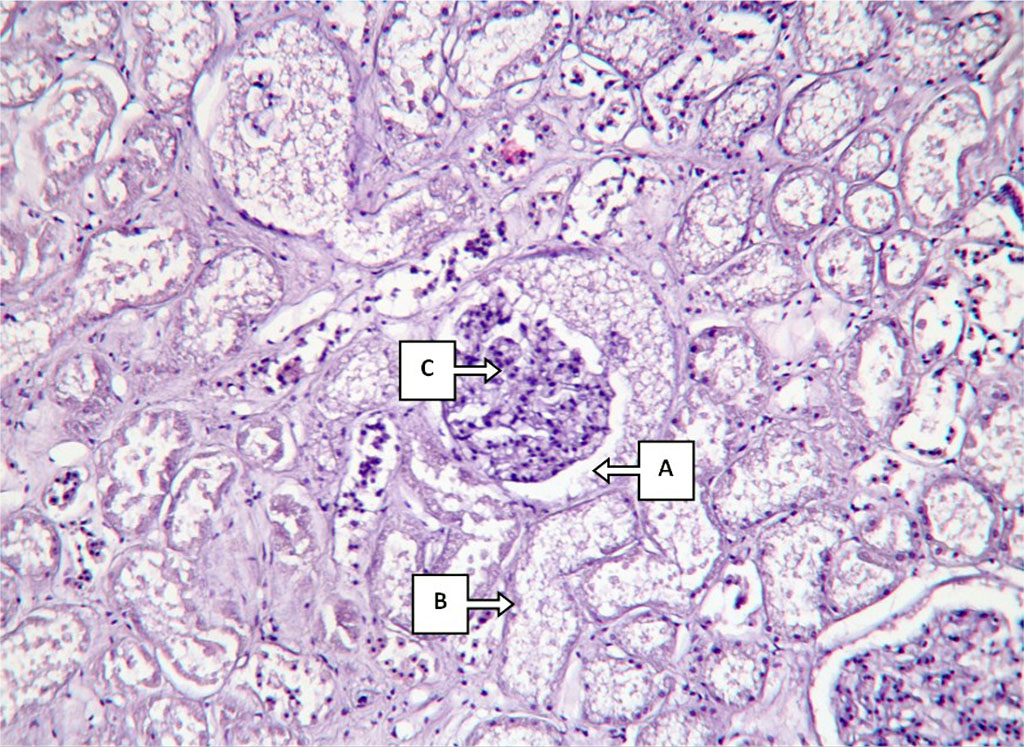
Fig.2. Histological changes in kidney structure in a 2-month antimony intoxication. Description in the text. Hematoxylin-eosin staining, 80x200x400
Heavy metals are known to have been used quite extensively in human use. Some of them have been used as medicines, for example, preparations of pentavalent antimony have been used in the treatment of leishmaniasis for more than 60 years [13]. Antimony has also been used to treat syphilis. Mercury-based antiseptics and diuretics have been produced [14]. Chromium preparations have a beneficial effect on glycemic levels in diabetic patients [15]. The nephro-, cardio- and hepatotoxic effects of some heavy metals have been previously studied in our laboratory. Models have been developed and effective doses of lead, nickel, copper and other metal compounds have been studied to simulate renal pathology, cardiovascular system, liver pathology and hemostasis system, and ways to prevent the pathogenic effects of metal pollutants are being developed [16,17,18]. Currently, there is enough information about the toxic effects of heavy metals, which casts doubt on the feasibility of their use, and therefore their use in medicine is declining.
The results of the experiments revealed that intragastric injection of antimony chloride daily for 2 months in rats leads to toxic nephropathy, which is characterized by lesions in the glomerular and tubular renal apparatus, electrolyte-and water excretion function of the kidneys, proteinuria. Manifestations of nephropathy after one month and two months of intoxication differ in direction, but are accompanied by pronounced proteinuria.